Heat Radiation
On the evening when Planck first reported the formula to Rubens, he did so on a postcard that was received by Rubens the next morning. After one or two days had passed, Rubens visited Planck and informed him that the new formula was in perfect agreement with his observations.

Reproduction of figure 2 on paper Über die Emission langwelliger Wärmestrahlen durch den schwarzen Körper bei verschiedenen Temperaturen by H. Rubens and F. Kurlbaum, published in Sitzungsberichte der Königlich Preussischen Akademie der Wissenschaften zu Berlin on 25 October 1900 (english translation provided by the Astrophysical Journal ). Note that Planck proposed his formula on 19 October 1900. Rubens and Kurlbaum discussed (and praised) Planck's formula in their paper, but they added it to the figures only in a successive paper on 1901. Worth to mention thar the curves in the figure are a linear transformation of the energy: a · E(λ, T) + b.
The birth of quanta
Sunday 7 October 1900 Rubens together with his wife visited Planck, the discussion turned to the measurements with which Rubens was occupied. Rubens said that for the longest wave-lengths — which he could achieve — the law recently proposed by Lord Rayleigh was valid. On receiving this information from Rubens, Planck set down and studied the theoretical implications for the equilibrium entropy, deriving the radiation formula that carries his name.The text has been adapted from the book The Historical Development of Quantum Theory. Vol. 1. by Mehra and Rechenberg.
The same evening Planck reported the formula to Rubens on a postcard, which the latter received the following morning. One or two day later Rubens went to Planck, and was able to bring him the news that the new formula agreed perfectly with his observations.Plank published his formula on paper Über eine Verbesserung der Wienschen Spektralgleichung the next 19 October.
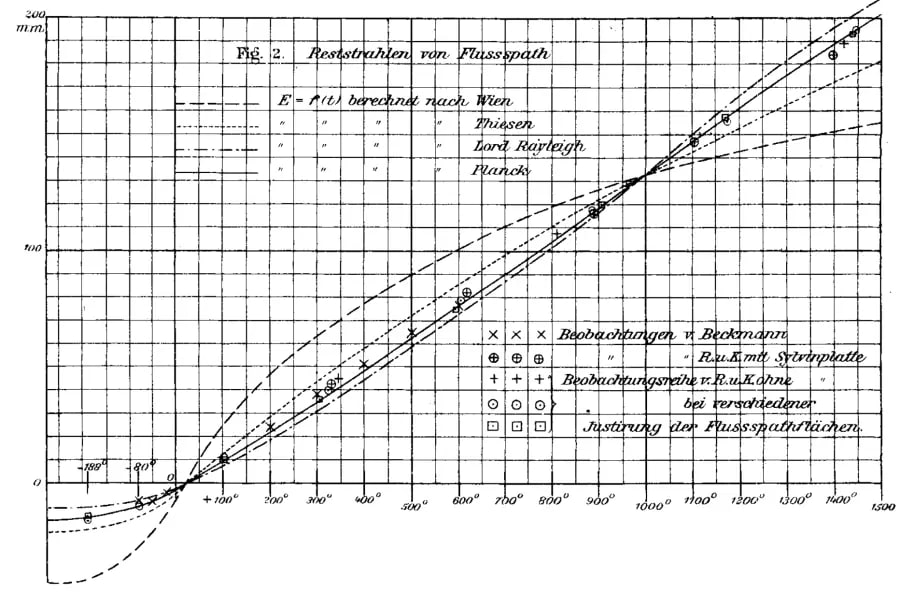
This image is the Figure 2 in the paper Anwendung der Methode der Reststrahlen zur Proefung des Strahlungsgesetzes by the H. Rubens and F. Kurlbaum, published one year later with the Planck's formula. Note that Planck proposed the formula in his paper on 19 October 1900. Rubens and Kurlbaum discussed Planck's formula in their first paper, but they added it to the figures only in the second paper.
Max Planck
1858-1947
Max Planck was a visionary German physicist renowned for revolutionizing the field of quantum mechanics. His groundbreaking work on the blackbody radiation spectrum laid the foundation for modern physics and earned him the Nobel Prize in Physics in 1918.
In the late 19th century, the prevailing view of radiation's behavior clashed with experimental observations. Planck approached this problem by introducing the radical notion of quantization, proposing that energy exchange occurred in discrete units or "quanta". To explain the blackbody radiation curve, he ingeniously derived a formula that accurately matched experimental data. This formula, now known as Planck's radiation law, revolutionized our understanding of how energy is emitted and absorbed by matter.
Planck's contributions not only established the theoretical framework for modern physics but also laid the groundwork for subsequent generations of physicists to delve deeper into the realm of the subatomic and reshape the landscape of scientific thought.

