Lines of Force
Michael Faraday, a pioneering 19th-century scientist, introduced groundbreaking concepts in electromagnetic science. Envisioning charged objects or magnets as sources of influence, he conceptualized invisible lines of force permeating space, visually depicting what we now recognize as a force field.

Artistic reproduction of the delineation of lines of magnetic force by iron filings.
A mathematician of a very high order
Michael Faraday introduced the notion of lines of force as a conceptual framework to elucidate the dynamics of what we today call electric and magnetic fields. As a visualization tool, lines of force facilitated a qualitative understanding the invisible forces governing the interactions between charged particles and magnets. While Faraday's approach was more qualitative than quantitative, it provided an intuitive grasp of these phenomena.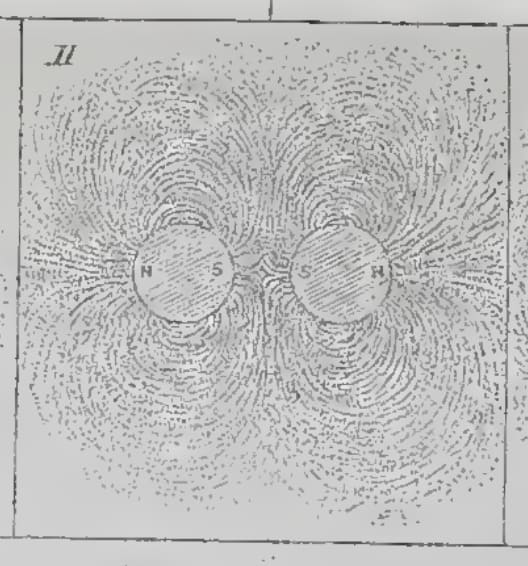
The concept of lines of force, introduced by Faraday, laid the groundwork for the subsequent development of field theory in physics. Fields, as fundamental entities, became integral to the work of later physicists, notably James Clerk Maxwell, who formulated the equations governing electromagnetic fields. Faraday's imaginative representation of lines of force thus played a crucial role in advancing our comprehension of the fundamental forces shaping the physical world.
No better words than the ones written by Maxwell can explain the importance of Faraday's idea:The geometry of position is an example of a mathematical science established without the aid of a single calculation. Now Faraday's lines of force occupy the same position in electro-magnetic science that pencils of lines do in the geometry of position. They furnish a method of building up an exact mental image of the thing we are reasoning about. The way in which Faraday made use of his idea of lines of force in co-ordinating the phenomena of magneto-electric induction shews him to have been in reality a mathematician of a very high order—one from whom the mathematicians of the future may derive valuable and fertile methods.
The previous text is part of an peper published by Maxwell in Nature, and collected onThe Scientific Papers of James Clerk Maxwell Volume 2, by Maxwell J.C., Niven W.D.
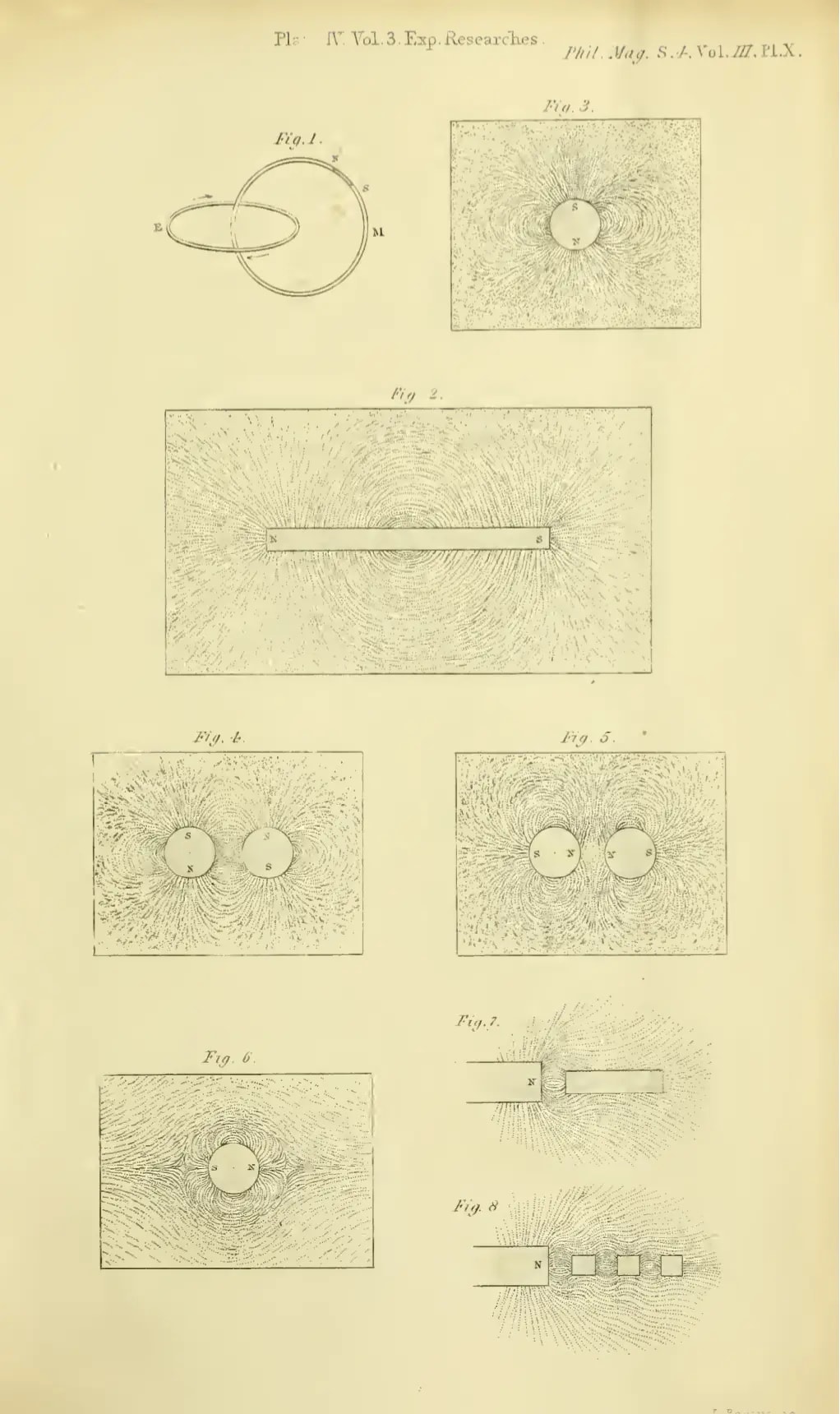
Experimental Researches in Electricity, § 37 Delineation of Lines of Magnetic Force by iron filings , note 3234: It would be a voluntary and unnecessary abandonment of most valuable aid, if an experimentalist, who chooses to consider magnetic power as represented by lines of magnetic force, were to deny himself the use of iron filings. By their employment he may make many conditions of the power, even in complicated cases, visible to the eye at once; may trace the varying direction of the lines of force and determine the relative polarity; may observe in which direction the power is increasing or diminishing; and in complex systems may determine the neutral points or places where there is neither polarity nor power, even when they occur in the midst of powerful magnets. By their use probable results may be seen at once, and many a valuable suggestion gained for future leading experiments.
Michael Faraday
1791 – 1867
Michael Faraday, born on September 22, 1791, in Newington Butts, Surrey, England, came from a humble background. With limited formal education, he began an apprenticeship as a bookbinder at a young age. His interest in science was sparked when he attended lectures by Sir Humphry Davy at the Royal Institution in London in 1812. Faraday's enthusiasm impressed Davy, who hired him as a laboratory assistant in 1813.
Faraday's scientific journey unfolded at the Royal Institution, where he made significant contributions to physics and chemistry. In 1821, he discovered electromagnetic rotation, a phenomenon that laid the groundwork for the development of the electric motor. Faraday observed that when a current-carrying conductor is placed in a magnetic field, it experiences a force that causes it to rotate. This discovery was a crucial step in understanding the relationship between electricity and magnetism, ultimately leading to the development of various electrical devices and technologies.
In the 1830s, Faraday formulated the laws of electrolysis, which describe the quantitative relationships between the amount of material produced or consumed during an electrolytic reaction and the amount of electric charge passed through the electrolyte. Faraday's work laid the foundation for modern electrochemistry, and played a vital role in the later development of concepts like Avogadro's law and the mole.
Despite his groundbreaking work, Faraday remained financially modest, declining honors and a knighthood. In 1860, Queen Victoria granted him a residence at Hampton Court in acknowledgment of his scientific contributions. Faraday retired from active research in the 1850s but continued lecturing and mentoring.

